

Organic chemistry is often the most difficult class that premeds must defeat on their way to medical school. But, you’ve now finished organic chemistry and are beginning to study for the MCAT. How much of this dreaded subject will come back to haunt you on the most important exam you’ll take as a premed?
Thankfully, organic chemistry won’t be a large portion of your exam. In fact, the MCAT will ask you only about 6 to 12 questions on organic chemistry out of 230 total questions! As a result, your study schedule should include a balance between content and practice. For organic chemistry questions, you’ll be able to increase your performance significantly by understanding how the test writers at the AAMC like to ask questions.
So, enjoy the practice passages we’ve designed for you here! We’ll test your chemistry knowledge using three MCAT-style passages and five standalone questions. Each explanation for the passage-based questions will have suggestions for what you should review if you miss a question. Good luck!
Fluorine-18 radiolabeling typically includes several conserved steps, which includes elution of [ 18 F] from an anion exchange cartridge with a basic solution of K2CO3 or KHCO3 and Kryptofix 2.2.2 in a mixture of acetonitrile and water followed by rigorous azeotropic drying to remove the water. Researchers attempted to develop a non-anhydrous and minimally basic (NAMB) technique to simplify the process and avoid basic conditions that can limit the scope and efficiency of [ 18 F]fluoride incorporation chemistry.
In a novel approach, researchers eluted [ 18 F]F− from small (10–12 mg) anion-exchange cartridges with solutions of tetraethylammonium bicarbonate, perchlorate, or tosylate in polar aprotic solvents containing 10–50% water. After dilution with additional aprotic solvent, these solutions were used directly in nucleophilic aromatic and aliphatic 18 F-fluorination reactions, which prevents the need for azeotropic drying.
As proof-of-principle, NAMB chemistry was utilized for the synthesis of the dopamine D2/D3 antagonist [ 18 F]fallypride. The standard synthesis of [ 18 F]fallypride is known to be base-sensitive, because of the tendency of the tosyl-fallypride precursor 6 to undergo hydrolysis and elimination side reactions, making this compound a good candidate with which to evaluate this minimally basic synthesis.
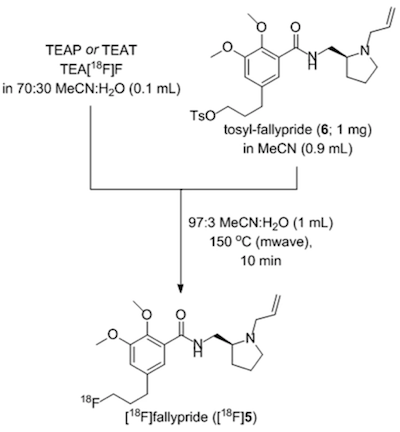
Figure 1. NAMB chemistry synthesis of [ 18 F]fallypride.
CREATOR AND ATTRIBUTION PARTY: INKSTER, J., AKURATHI, V., SROMEK A, ET AL. A NON-ANHYDROUS, MINIMALLY BASIC PROTOCOL FOR THE SIMPLIFICATION OF NUCLEOPHILIC 18F-FLUORINATION CHEMISTRY. SCI REPORTS 10, 6818 (2020). THE ARTICLE’S FULL TEXT IS AVAILABLE HERE: https://www.nature.com/articles/s41598-020-61845-y. THE ARTICLE IS NOT COPYRIGHTED BY SHEMMASSIAN ACADEMIC CONSULTING. DISCLAIMER: SHEMMASSIAN ACADEMIC CONSULTING DOES NOT OWN THE PASSAGE PRESENTED HERE. CREATIVE COMMON LICENSE: HTTP://CREATIVECOMMONS.ORG/LICENSES/BY/4.0/. CHANGES WERE MADE TO ORIGINAL ARTICLE TO CREATE AN MCAT-STYLE PASSAGE.
1. In a novel experiment, researchers find a new [ 18 F]fluoride that can carry out NAMB chemistry upon electron capture. Which of the following best describes the element after positron emission?
A) [ 18 F]fluoride
B) [ 17 F]fluoride
2. Researchers discover that high concentrations of a protic solvent in a new experiment prevent nucleophilic aromatic 18 F-fluorination reactions necessary for product formation. Which of the following best explains this observation?
A) Protic solvents will hydrogen bond with nucleophiles, thereby decreasing nucleophilicity.
B) Protic solvents will hydrogen bond with electrophiles, thereby decreasing electrophilicity.
C) Protic solvents will form Van der Waals interactions with aromatic rings, thereby decreasing nucleophilicity.
D) Protic solvent will form dipole-dipole interactions with electrophiles, thereby decreasing electrophilicity.
3. Which of the following functional groups present in compound 6 is important for the hydrolysis side reaction?
4. Researchers are having trouble obtaining pure compound 6. They hypothesize that protonating the amide nitrogen in compound 6 will allow them to use which of the following columns for purification?
A) Anion-exchange because the positive charge on 6 will bind the negatively charged resin.
B) Anion-exchange because the negative charge on 6 will bind the positively charged resin.
C) Cation-exchange because the positive charge on 6 will bind the negatively charged resin.
D) Cation-exchange because the negative charge on 6 will bind the positively charged resin.
Answers and Explanations for MCAT Organic Chemistry Practice Passage #1
1. Answer choice C is correct. The balanced equation would appear as follows:
[ 18 9F]fluoride → 0 +1β + [ 18 8O]oxygenTo solve radioactive decay problems, you must ensure that the top line (18 + 0 = 18) and the bottom line (8 + 1 = 9) are equal to one another (choice C is correct; choices A, B, and D are incorrect).
Review radioactive decay.
2. Answer choice A is correct. Protic solvents hydrogen bond with nucleophiles, thereby decreasing nucleophilicity and preventing the reaction of interest from occurring (choice A is correct; choice C is incorrect). Electrophilicity is not affected by protic solvents in this case (choices B and D are incorrect).
Review nucleophilicity and electrophilicity.
3. Answer choice C is correct. An amide group can undergo hydrolysis through nucleophilic acyl substitution (choice C is correct). An ether (R-OR’) is present on the molecule, but it is unlikely to undergo hydrolysis (choice A is incorrect). An amine is also unlikely to undergo hydrolysis (choice D is incorrect). Though an ester group will be more susceptible to hydrolysis than an amide, this functional group is not present in Compound 6 (choice B is incorrect).
Review functional groups and nucleophilic acyl substitution.
4. Answer choice C is correct. Protonation of the amide nitrogen would lead to an additional positive charge (choices B and D are incorrect). An anion-exchange column binds negatively charged molecules, meaning its resin (which is in the column itself) will be positive (choice A is incorrect). The cation-exchange column binds positively charged molecules, and its resin will therefore be negative (choice C is correct).
Review protonation and column chromatography.